Contact lenses worn on a daily wear schedule need to be stored overnight, and they must be stored in a solution that disinfects the lenses to remove colonising microbes from the lens surface resulting in a non-contaminated lens to be worn on the following day. In addition to disinfecting the lenses overnight, solutions also clean lenses of contaminating tear film components (proteins/lipids) from the lens surface.
There are basically two groups of contact lens disinfecting/cleaning solutions available for use with soft lenses (including silicone hydrogels). The so-called multipurpose disinfecting solutions (MPDS) contain biguanides in one form or another or polyquaternium-1. They may also contain other disinfectants such as myristamidopropyl dimethylamine. All these agents have the same general mode of action; disruption of the microbial membranes that ultimately leads to death of the microbes. The second group is essentially one entity – hydrogen peroxide (H2O2). H2O2 is a strong oxidising agent and can disrupt many aspects of a microbe – acting on proteins, lipids, DNA – again leading to cell death. To achieve effective lens cleaning, disinfecting solutions may also contain a variety of surfactants.
The MPDS and H2O2 solutions have varying abilities to control microbial growth, but they can also interact with the cornea/conjunctiva in different ways that affect overall clinical performance. Components of solutions can be transferred into the eye and certain compounds from the solutions will adsorb to lenses during the overnight storage. Thus, balance needs to be maintained between disinfectant activity and “toxicity” that may manifest during wear in several ways. These two aspects, disinfection and clinical performance, will be discussed in the current article.
The disinfecting efficacy of any of these solution types is regulated by the International Organisation for Standardisation – ISO and the solutions have to pass various standard tests before being marketed. All commercially available solutions must have passed these tests, and perhaps other tests in different countries around the world, prior to commercialisation. Even then, certain solutions have been shown to “fail” during wearer use, and I have discussed this previously on this site (http://www.siliconehydrogels.org/editorials/07_aug.asp). Here, I will compare the anti-microbial efficacy of these solution groups.
If tests are performed comparing MPDS with H2O2 in the absence of neutralisation of the peroxide, almost always the H2O2 systems show greater microbial killing than the MPDS systems (Stapleton et al., 1998; Hughes et al., 2003). However, this isn’t a relevant comparison. For so-called one-step H2O2 solutions, there is a built in catalyst (platinum) that converts the H2O2 to water over time. Two-step H2O2 solutions require the addition of a neutralising agent after a recommended disinfection time (4-6 hours). Two-step H2O2 solutions are very efficient disinfecting solutions, for example, killing even large inocula of Acanthamoeba cysts (a known resistant form of this amoeba; Hiti et al., 2005). One-step solutions on the other hand are either less effective (Miller et al., 2001), especially against Acanthamoeba cysts (Hiti et al., 2005; Cengiz et al., 2000), or allow the re-growth of microbes upon prolonged storage (Rosenthal et al., 1999). Indeed, one-step H2O2 solutions are probably only as effective as the MPDS, with the added disadvantage that prolonged storage of lenses in the neutralised solution (effectively saline/water) can promote re-growth of microbes. Prolonged storage of lenses might occur with occasional lens wearers (perhaps people wearing lenses as fashion accessories). MPDS disinfectants do not decompose and therefore provide continued disinfection activity. Furthermore, a study from 1995 demonstrated that use of one-step H2O2 (only a few subjects used two-step H2O2) was associated with increased levels of microbial contamination of contact lens storage cases compared to a mixed bag of non-peroxide chemical disinfecting solutions (Gray et al., 1995), and the microbes that were contaminating the cases were catalase producers (an enzyme that inactivates peroxide). A study of microbial keratitis (MK) in Hong Kong reported use of one-step H2O2 was three times more likely to be associated with MK compared to use of MPDS. However this latter study might be confounded if the people using one-step were not everyday wearers.
From the data available and from an antimicrobial disinfection perspective, use of two-step H2O2 solutions should be the solution of choice. It’s a pity that in many countries these are no longer widely available!
H2O2 solutions are not without other disadvantages. Many users inadvertently fail to neutralise H2O2 or perhaps use the solution to wash lenses and suffer the pain and trauma of putting H2O2 into their eyes! Also, the extra procedures needed with two-step H2O2 probably make these solutions fall out of favour. Morgan and Efron (2006) reported that, in the UK, the proportion of wearers using two-step H2O2 solutions reduced from 1997 to 2005, when it was <5%. Similarly, use of even one-step H2O2 reduced over the same period. MPDS solutions, on the other hand, showed a steady increase in use, representing around 90% of the market in 2005.
The clinical performance of H2O2 solutions versus MPDS has recently received greater scrutiny. This has been brought to the forefront by various people, notably Andrasko and our research group at the Institute for Eye Research (IER). Following the report by Jones et al. in 2002 of an association between an MPDS and a silicone hydrogel lens showing “asymptomatic corneal staining”, both Andrasko and IER have produced grids demonstrating that other MPDS/silicone hydrogel combinations can cause similar staining (referred to by IER as Solution Induced Corneal Staining; Figure 1)
Figure 1. Comparison of Andrasko and IER grids of Solution Induced Corneal Staining (see http://www.ier.org.au/news/0408/news1.asp).
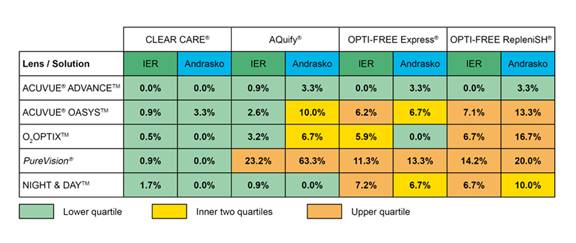
It is evident from Figure 1 that, in general, use of the H2O2 solution (Clear Care) results in the lowest level of SICS compared to any of the MPDS solutions, irrespective of the silicone hydrogel lenses worn by subjects. The level of SICS has been correlated in some studies with reduced comfort of lenses during wear (Papas et al., 2008), although others have not found this correlation (Garofalo et al., 2005).
Carnt et al. (2007a), have reported that asymptomatic corneal infiltrates are associated with SICS; subjects presenting with SICS are three to six times more likely to have had corneal infiltrates. This implies a possible causative effect. However, further statistical analysis did not support a causative effect, as it was shown that upon removing from the analysis those subjects who had SICS, corneal infiltrates were still more common with users of MPDS compared to H2O2 (Carnt et al., 2007b). This does, however, implicate MPDS in the production of infiltrates.
Nichols (2006), has shown that using a rub step in the lens cleaning regime with MPDS results in approximately the same level of clinical deposits on lenses as when a H2O2 solution is used with a protein removal rub step.
In conclusion, the data presented indicate that use of two-step H2O2 would probably be most protective for the user as these solutions offer the best antimicrobial activity. Unfortunately from a marketing perspective these solutions are essentially obsolete. Whilst there are differences in both microbiological and clinical performance of one-step H2O2 and MPDS, they are both relatively safe, and it is probably largely a matter of determining how frequently a wearer will wear their lenses (or in other words how long they might store their lenses) as to which solution to recommend. Perhaps also, further data on the relation between MPDS and asymptomatic infiltrates might further help the practitioner and lens wearer to make informed choices.
References
- Stapleton F. Harmis N. Deshpande R. Tran D. 1998. Preliminary studies on the amoebicidal efficacy of contact lens disinfection systems. Australian & New Zealand Journal of Ophthalmology. 26 Suppl 1:S44-6.
- Hughes R, Heaselgrave W, Kilvington S. 2003. Acanthamoeba polyphaga strain age and method of cyst production influence the observed efficacy of therapeutic agents and contact lens disinfectants. Antimicrobial Agents and Chemotherapy. 47; 3080–4.
- Hiti K. Walochnik J. Faschinger C. Haller-Schober E-M. Aspock H. 2005. One- and two-step hydrogen peroxide contact lens disinfection solutions against Acanthamoeba: How effective are they? Eye 19: 1301-5.
- Rosenthal RA. Buck S. McAnally C. Abshire R. Schlech B. 1999. Antimicrobial comparison of a new multi-purpose disinfecting solution to a 3% hydrogen peroxide system. CLAO Journal. 25:213-7.
- Miller MJ. Callahan DE. McGrath D. Manchester R. Norton SE. 2001. Disinfection efficacy of contact lens care solutions against ocular pathogens. CLAO Journal. 27:16-22.
- Cengiz AM. Harmis N. Stapleton F. 2000. Co-incubation of Acanthamoeba castellanii with strains of Pseudomonas aeruginosa alters the survival of amoeba. Clinical and Experimental Ophthalmology 28: 191–3.
- Morgan P. Efron N. 2006. A decade of contact lens prescribing trends in the United Kingdom (1996–2005). Contact Lens & Anterior Eye 29: 59–68.
- Jones L. MacDougall N. Sorbara LG. 2002. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optometry & Vision Science. 79:753-61.
- Garofalo RJ. Dassanayake N. Carey C. Stein J. Stone R. David R. 2005. Corneal staining and subjective symptoms with multipurpose solutions as a function of time. Eye & Contact Lens 31: 166–74.
- Papas EB. Carnt N. Willcox MDP. Holden BA. 2008. Complications associated with care product use during silicone daily wear of hydrogel contact lens. Eye & Contact Lens 33: 392–393
- Gray TB. Cursons RTM. Sherwan JF. Rose PR. 1995. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. British Journal of Ophthalmology. 79: 601-5.
- Carnt N. Jalbert I. Stretton S. Naduvilath T. Papas E. 2007a. Solution toxicity in soft contact lens daily wear is associated with corneal inflammation. Optometry & Vision Science. 84:309-15.
- Carnt NA. Keay L. Naduvilath T. Holden B. Willcox M. 2007b. Risk factors associated with corneal inflammation in soft contact lens daily wear. 4326/B762. Association for Research in Vision and Ophthalmology. Fort Lauderdale, USA.
- Nichols JJ. 2006. Deposition rates and lens care influence on galyfilcon a silicone hydrogel lenses. Optometry and Vision Science 83:751–7.
|