The advent of the silicone hydrogel lenses has presented new challenges in care. Lenses have evolved from rigid to rigid gas permeable (RGP) lenses to provide oxygen to the cornea. Neither contained water, but used smaller lenses to provide exchange of the tears for needed nutrition and removal of waste products. Larger conventional hydrogel lenses were developed based primarily on water loving (hydrophilic) ingredients and use water to transport necessary ions to the cornea. In conventional hydrogels, oxygen available to the cornea is limited by transport through water. Given the diverse approaches to the lens materials, different product regimens were developed for their care. Different criteria for selection of the individual lens material chemistries of rigid and soft lens materials evolved for evaluation of care systems. The rigid gas permeable lenses, providing oxygen through the lens, were divided into a series of four groups based on their chemistries1(Table 1).
Table 1
Rigid Hydrophobic Lenses
(Less than 10% Water)
Group |
Description |
I |
Materials not containing silicon or fluorine |
II |
Materials containing silicon but not fluorine |
III |
Materials containing silicon and fluorine |
IV |
Materials containing fluorine but not silicon |
The conventional hydrogels were also divided into four groups based on their interactions with the environment and care systems (Table 2). [1,2,3,4]
Table 2
Soft Hydrophilic Lenses
Group |
Description |
I |
Low Water Content (<50%), Nonionic* |
II |
High Water Content (> 50%), Nonionic |
III |
Low Water Content (<50%), Ionic** |
IV |
High Water Content (> 50%), Ionic |
* < 1% mole fraction ionic content at pH= 7.2
** > 1% mole fraction ionic content at pH= 7.2 |
The continuum of contact lenses was able to be described by two basic lens technologies and eight different approaches to classifying the chemistry of the materials. These systems were developed so that lens materials could be tested with a representative lens material from each group with the reasonable assurance that the care system would be able to cover the full range of available lens materials in that group. In the early 1980’s, before grouping of lenses, the care system had to test and list each acceptable lens brand on its packaging.
Now, a new modality, the silicone hydrogels, combining the attributes of rigid gas permeable lenses and the conventional hydrophilic lens technologies challenges the ability to care for contact lenses. The silicone hydrogel lenses enhance the ability to provide the oxygen requirement of the cornea and include a hydrophilic domain to provide the transport of the micronutrients and removal of waste through the larger traditional hydrogel lens designs. The initial approach was to use the existing classification schemes [1,2,3,4] developed for rigid and hydrophilic or traditional hydrogel lenses. Consideration of the silicone hydrogel in the rigid gas permeable scheme that closely mirror the oxygen properties of silicone hydrogels was eliminated by the inclusion of greater than 10% water. Using the conventional hydrogel classification groups, the silicone hydrogels were determined to fall into groups 1 (nonionic, water content less than 50%) and group 3 (ionic, water content less than 50%). It must be noted that the initial development of the hydrogel classification system ignored the influence of the hydrophobic monomers since those hydrophobic monomers were presence in very low concentrations. [5]
Careful examination of the properties of the conventional hydrogel materials in the four groups of soft, hydrophilic contact lenses and the new silicone hydrogels reveal striking differences in the properties of these materials.
The most obvious difference is oxygen permeability represented by the high silicone content of these materials. The contrast is shown in Figure 1 abstracted from K. Dumbleton’s editorial Oct. 2002 from www.siliconehydrogels.org. [6]
Figure 1 |
Comparison of Dk of Conventonal Hydrogels and Silicone Hdrogels |
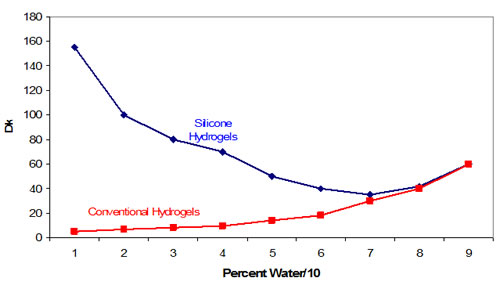 |
The association of lysozyme, the principal protein associated with lenses, does not show significant differences between Group 1 low water, nonionic lenses and silicone hydrogels; however, the percent of protein denatured on the contact lenses is significantly higher on silicone hydrogel lenses when compared to ionic group 4 contact lens. [7] While the lipid association with lenses has been reported with somewhat different results, [9,10] the appearance of non-wetting areas can easily be seen in the slit lamp. Measurements and differences of wettability have been reported based on preclinical and ex-vivo wetting measurements.11 Clinical differences have also been reported by Jones comparing different lens care systems on PureVision lenses [12], and by Andrasko [13] in testing products and lenses.
Given the significant differences in properties between silicone hydrogels and either rigid gas permeable materials or conventional hydrogels, a new and separate classification system is needed for the silicone hydrogels. Stone [14] provided the initial approach to classification in the May 2007 issue of Cornea and Contact Lenses. The initial approach was based on the observation that with the exception of one material the oxygen content was inversely correlated to the water content of the initial five silicone hydrogel materials developed with a correlation (r2) of 0.96. (Figure 2) This observation was based on the Dk and water content reported for the available materials.
Figure 2 |
Correlation of Oxygen Permeability and Water Content for Five Silicone Hydrogel Lenses |
|
The remaining material, Comfilcon A, was distinctly different reducing the correlation (r2) equal to 0.49. (Figure 3)
Figure 3 |
Correlation of Oxygen Permeability and Water Content for Six Silicone Hydrogel Lenses |
|
These initial results suggest differences in achieving both the silicone architecture and the inclusion of the hydrophilic portion. This becomes the first criteria for defining the current silicone hydrogel lens materials.
The second approach to the classification of silicone hydrogels examines the hydrophilic portion. The primary approach to building the silicone hydrogel polymer hydrophilic structure has been based on n-vinyl pyrrolidinone, a non-ionic amide structure. The use of ionic monomers in conventional hydrogels has been a primary distinguishing feature with Group 4 hydrogel materials showing significantly different interactions with proteins [15] and significant uptake of cationic preservatives. [16,17] One material, Balafilcon, also contains a vinyl substituted amino acid which provides ionic character to the monomer and the resulting polymer. These two base characteristics provide a method to separate Comfilcon and Balafilcon from the remaining silicone hydrogel materials.
The four remaining materials have used two different approaches to achieve a wettable surface suitable for use in contact lenses. The Lotrafilcon A and B materials use a plasma treatment in the presence of glycols to achieve a hydrophilic surface layer. Plasma treatment without the presence of a chemical additive is also used to chemically modify the surface of the balafilcon material, although it is anticipated that the effect of the ionic monomer provides the primary mechanism for differentiation. The remaining silicone hydrogel materials gain wettable surfaces by the specific inclusion of a wetting polymer. The release or migration to the surface of these hydrophilic ingredients provides wettability and defines the galyfilcon and senofilcon polymers.
Combining these characteristics we can currently describe the silicone hydrogels as a separate set of polymers which have been described as “Group 5 Hydrophilic (Soft) Contact Lenses.” [14] To use the differences to design and select care systems, the materials can be separated into the subgroups shown in Table 3.
Table 3
Classification of Hydrogels Containing Silicon
(Group 5)14
A |
B |
C |
D |
Non-Linear Relationship of Dk to Water Content |
Contains an Ionic Component |
Has a Plasma or Bonded Surface Modification |
Contains a “releasing” wetting agent |
Comfilcon A |
Balafilcon A |
Lotrafilcon A
Lotrafilcon B |
Galyfilcon A
Senofilcon |
It took nearly fifteen years of experience with conventional hydrogels before we finalized a classification system that could fully describe the conventional hydrogels.
This classification approach is based on current data. Other proposals have surfaced in discussions at ANSI and ISO based on other parameters including pore size and descriptions of the chemical composition, and new silicone materials are on the horizon. We are now about seven years into understanding the science of silicone hydrogels in the marketplace, and using lens care technologies, many originally developed for care of conventional hydrogels. Only recently have any products appeared tested specifically for the silicone hydrogel lens. This approach to classification will be tested by the development of new materials and care systems.
U.S. FDA Premarket Notification (510(k)) Guidance Document for Daily Wear Contact Lenses, June 28,1994.
- U.S. FDA Draft Testing Guidance for Class III Soft Contact Lenses, 1988:16
- U.S. FDA Premarket Notification (510(k)) for Lens Care Products, May 1, 1997.
- Stone, R.P., Lens Groups and Lens Care, Journal of the Japan Contact Lens Society, 1997; 39:32-38.
- Stone, R.P., Why Contact Lens Groups, Contact Lens Spectrum, 1988: 38-41
- Dumbleton, K. The Physical and Clinical Characteristics of Silicone Hydrogel Lenses: How they Work, www.siliconehydrogels.org, Oct 2002.
- Suwala, M et al. Quantity and Conformation of Lysozyme Deposited on Conventional and Silicone Hydrogel Contact Lenses Using an In Vitro Model, Eye and Contact Lens 33(3): 138-143, 2007
- Minno, G., Quantitative Analysis of Protein Deposits on Hydrophilic Soft Contact Lenses: I. Comparison to Visual Methods of Analysis. II. Deposit Variation among FDA Material Lens Groups, Optom. Vis. Sci. 1991; 68: 865-872
- Jones, L. et al., Lysozyme and Lipid Deposition on Silicone Hydrogel Lens Materials, Eye and Contact Lens 2003; 29:S75-S79
- Maziarz, EP, et al., Lipid deposition on Silicone Hydrogel Lenses: Quantification of Oleic Acid, Oleic Acid Methyl Ester and Cholesterol, Eye and Contact Lens 2006; 32(6): 300-307
- Ketelson, H., et al., Wettability of Silicone Hydrogel Lenses in the Presence of Tear Components, Review of Cornea and Contact Lenses, April 2005: 24-28
- Jones, L. et al., Asymptomatic Corneal Staining Associated with the Use of Balafilcon Silicone-Hydrogel Contact Lenses Disinfected with a Polyaminopropyl Biguanide-Preserved Care Regimen, Optom. Vis. Sci. 2002; 79(12): 753-761.
- Andrasko, G, Poster presented at ARVO 2005, Presented at BCLA 2007, and current status of research found at www.staininggrid.com
- Stone, R.P., Classifying Silicone Hydrogel Materials, Review of Cornea and Contact Lenses May 2007:26-30
- Minno, G. et al., Quantitative Analysis of Protein Deposits on Hydrophilic Contact Lenses : I Comparison to Visual Methods of Analysis. II Deposit Variation Among FDA Lens Material Groups, Optom. Vis. Science 1991 ; 68(11) : 865-872
- Schlitzer, R, Preservative Uptake by Soft Contact Lenses, Contact Lens Spectrum 1992: 41-43
- Rosenthal, R, et al., Loss of Bacterial Activity from Contact Lens Solutions, CLAO J. 1997; 23(1): 57-62
|