| Silicone-hydrogel contact lens materials represent
a new family of biomaterials, whose properties are unlike any
other previously developed for contact lens use. The incorporation
of siloxane groups into the base hydrogel material has produced
materials that have substantially improved oxygen transmission
characteristics compared with conventional soft contact lens materials
1-3 and the results from clinical studies conducted to-date indicate
that the number of physiological complications induced by the
overnight use of such materials is significantly less than that
seen with conventional materials. 4-11
Detailed explanations of the development of silicone hydrogel
materials for contact lenses and their polymer chemistry have
been described in detail elsewhere. 2, 12-16
The use of silicon-containing flexible contact lenses is not new,
as silicone-elastomeric lenses have been used for therapeutic
and paediatric applications for many years. 17
These lenses offer exceptional oxygen transmission, but the migration
of siloxane moieties to the material surface results in the production
of extremely hydrophobic surfaces, resulting in marked lipid deposition.
18 To overcome this, the surfaces
of the two commercially available silicone-hydrogel lenses are
surface treated, 2, 13,
19, 20 in an attempt
to improve the wettability of the materials and to reduce the
degree of deposition that would occur on non-treated materials.
The surfaces of Focus Night & Day (lotrafilcon) lenses are
permanently modified in a gas plasma reactive chamber to create
a permanent, ultrathin (25nm), high refractive index, continuous
hydrophilic surface. 13, 21
PureVision (balafilcon) lenses are surface treated in a gas plasma
reactive chamber which transforms the silicone components on the
surface of the lenses into hydrophilic silicate compounds. 2,
19, 20 Glassy, discontinuous
silicate “islands” result, 19,
20 and the hydrophilicity of these areas
"bridges" over the underlying hydrophobic balafilcon
A material.
The deposition of contact lenses with substances derived from
the tear fluid is a well-known clinical complication, resulting
in reductions in comfort, 22 vision 23
and increased inflammatory responses. 24
Hydrogel materials rapidly spoil with constituents from the tear
film, particularly proteins, 25, 26
lipids 27 and mucins. 28
The adsorption of proteins and lipids at the contact lens interface
is dependent upon a number of factors. Notable amongst these are
material water content, 29, 30
surface charge, 29, 31,
32 wearing period 33
and age of the lens material. 34
Increasing water content and/or ionicity of the lens material
greatly enhances protein deposition, 25,
29-32, 35-37
with lysozyme being detectable on FDA group IV lenses after wearing
times for as little as one minute. 38
Whilst group IV lenses tend to predominantly deposit lysozyme,
neutral group II lens materials (particularly those containing
vinyl pyrrolidone) have a tendency to deposit lipid. 33,
39, 40
Information concerning the degree of protein and lipid deposition
that occurs on silicone-hydrogel lens materials is of significant
clinical importance, as these lenses are intended for in-eye use
for up to 30 days without removal. It is imperative that materials
worn in this way deposit as little material from the tear film
as possible, in order to minimise the potential visual and inflammatory
complications detailed above.
Results from our group 41, 42
and others 43-45
indicate that the amount of lysozymedeposited on silicone-hydrogel
lens materials is significantly less than that seen on group IV
traditional contact lens materials. Figure 1 indicates that silicone-hydrogels
typically deposit less than 10 µg of lysozyme after being
worn in-eye for 30 days, as compared with an etafilcon-lens (Acuvue),
which deposits in the region of 1000 µg of lysozyme after
one week of extended-wear.
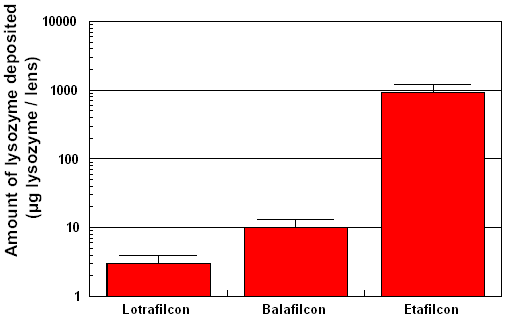 |
| Figure 1 Degree
of lysozyme deposition measured on Focus Night & Day (lotrafilcon),
PureVision (balafilcon) and Acuvue (etafilcon). The lotrafilcon
and balafilcon lenses were worn for 30 nights continuously
and the etafilcon lens was worn for 6 nights without removal.
The degree of lysozyme deposition was significantly different
between all three lens types (p<0.001). |
Despite surface treatment of the lens materials, in vitro wetting
angle assessment of the two silicone-hydrogel materials indicates
that they remain relatively hydrophobic compared with conventional
hydrogels. 44, 46, 47
Lipids preferentially deposit onto hydrophobic surfaces and data
thus far indicates that lipid deposition on silicone-hydrogels
can be a problem for certain patients. 41
Figure 2 reveals data from our laboratory, which indicates that
certain classes of lipids preferentially deposit onto silicone-hydrogel
lens materials and that silicone-hydrogels deposit greater quantities
of lipid than conventional ionic lens materials.
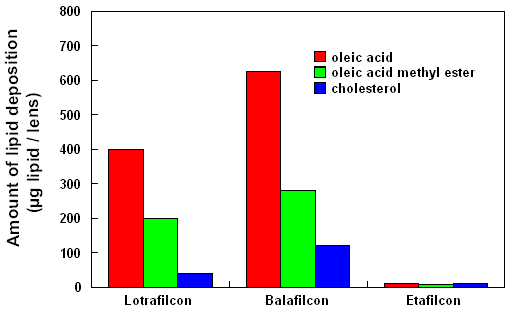 |
| Figure 2 Degree
of lipid deposition measured on Focus Night & Day (lotrafilcon),
PureVision (balafilcon) and Acuvue (etafilcon). The lotrafilcon
and balafilcon lenses were worn for 30 nights continuously
and the etafilcon lens was worn for 6 nights without removal.
The degree of lipid deposition was significantly different
between all three lens types (p<0.001). |
Lipid deposition onto conventional lens materials
is highly patient dependent 33, 34,
48-50 and this fact
is similarly observed with silicone-hydrogel lens materials. Figures
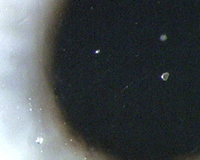
3 and 4 reveal various lipid deposition patterns seen in certain
subjects using silicone-hydrogel lenses.
 |
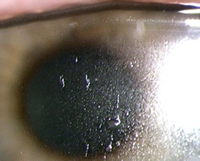 |
Figure 3 Lipid
deposition in the form of lens calculi (jelly-bumps) on a
silicone-hydrogel lens. Picture courtesy of Brian Tompkins.
|
Figure 4 Lipid
deposition in the form of a heavy film on the lens surface
of a silicone-hydrogel lens. Picture courtesy of Brian Tompkins. |
| Click
to enlarge |
Click
to enlarge |
If subjects are seen to be depositing their silicone-hydrogel
lenses with lipid then moving to non vinyl pyrrolidone-containing
materials (such as Proclear or Acuvue) should reduce this phenomenon.
Further options include adding surfactant cleaners containing
alcohol (such as Miraflow) or moving to more frequent periods
of replacement. 33
In summary, currently available, first-generation silicone-hydrogel
lens materials have provided clinicians with materials that allow
safe, oedema-free overnight wear for up to 30 continuous nights.
They deposit only small amounts of protein from the tear film,
but certain patients do have problems related to the deposition
of lipids on these materials, due to the relatively hydrophobic
nature of the lens surfaces. Subsequent iterations of siloxane-hydrogels
should be designed with surfaces that are ideally more hydrophilic,
in an attempt to optimise their biocompatibility with the tear
film.

Acknowledgements
We would like to acknowledge the work of Ian Forbes, Derek Louie,
Chris May and Jillian Schickler for their assistance in producing
the results published in this article.

|
|
1. Alvord L, Court J, Davis T, Morgan CF, Schindhelm K, Vogt J,
Winterton L: Oxygen permeability of a new type of high Dk soft contact
lens material. Optom Vis Sci 1998; 75: 30-6.
2.
Tighe B: Silicone hydrogel materials - how do they work ? in Silicone
Hydrogels: The Rebirth of Continuous Wear Contact Lenses, D. Sweeney,
Editor. Oxford, Butterworth-Heinemann,2000, pp 1 - 21.
3. Morgan CF, Brennan NA, Alvord L: Comparison of
the coulometric and polarographic measurement of a high-Dk hydrogel.
Optom Vis Sci 2001; 78: 19-29.
4. Brennan
NA, Coles ML, Comstock TL, Levy B: A 1-year prospective clinical
trial of balafilcon a (PureVision) silicone-hydrogel contact lenses
used on a 30-day continuous wear schedule. Ophthalmology 2002; 109:
1172-7.
5. Morgan PB, Efron N: Comparative
clinical performance of two silicone hydrogel contact lenses for
continuous wear. Clin Exp Optom 2002; 85: 183-92.
6.
Papas E, Vajdic C, Austen R, Holden B: High oxygen-transmissibility
soft contact lenses do not induce limbal hyperaemia. Curr Eye Res
1997; 16: 942-948.
7. Dumbleton KA, Chalmers
RL, Richter DB, Fonn D: Changes in myopic refractive error with
nine months' extended wear of hydrogel lenses with high and low
oxygen permeability. Optom Vis Sci 1999; 76: 845-9.
8.
Keay L, Sweeney DF, Jalbert I, Skotnitsky C, Holden BA: Microcyst
response to high Dk/t silicone hydrogel contact lenses. Optom Vis
Sci 2000; 77: 582-5.
9. Covey M, Sweeney
DF, Terry R, Sankaridurg PR, Holden BA: Hypoxic effects on the anterior
eye of high-Dk soft contact lens wearers are negligible. Optom Vis
Sci 2001; 78: 95-9.
10. du Toit R, Simpson
TL, Fonn D, Chalmers RL: Recovery from hyperemia after overnight
wear of low and high transmissibility hydrogel lenses. Curr Eye
Res 2001; 22: 68-73.
11. Sweeney D, Keay
L, Jalbert I, Sankaridurg P, Holden B, Skotnitsky C, Stephensen
A, Covey M, Rao G: Clinical performance of silicone hydrogel lenses.
in Silicone hydrogels. The rebirth of continuous wear contact lenses,
D. Sweeney, Editor. Oxford, Butterworth-Heinemann,2000, pp 90 -
149.
12. Kunzler J, Ozark R. Hydrogels
based on hydrophilic side chain siloxanes. in The American Chemical
Society Division of Polymeric Materials - Science and Engineering
Polym Mater Sci Eng Proc Acs Div, Polym Mater Sci Eng, ACS. 1993.
Chicago, Il, USA.
13. Nicolson PC, Vogt J: Soft contact lens polymers: an evolution.
Biomaterials 2001; 22: 3273-83.
14. Friends
G, Kunzler J, Ozark R: Recent advances in the design of polymers
for contact lenses. Macromolecular Symposia 1995; 98: 619-631.
15. Kunzler J: Silicone-based hydrogels for
contact lens applications. Contact Lens Spectrum 1999; 14: 9 - 11.
16. Dumbleton K: The
physical and clinical characteristics of silicone-hydrogel lenses:
How they work?" Silicone Hydrogel Website 2001.
17. Gurland JE: Use of silicone lenses in infants
and children. Ophthalmology 1979; 86: 1599-1604.
18.
Huth S, Wagner H: Identification and removal of deposits on polydimethylsiloxane
silicone elastomer lenses. Int Contact Lens Clin 1981: 19-26.
19. Grobe GL, 3rd: Surface engineering aspects
of silicone-hydrogel lenses. Contact Lens Spectrum 1999; 14: 14
- 17.
20. Lopez-Alemany A, Compan V, Refojo
MF: Porous structure of Purevision versus Focus Night&Day and
conventional hydrogel contact lenses. J Biomed Mater Res (Appl Biomat)
2002; 63: 319 - 325.
21. Weikart CM, Matsuzawa
Y, Winterton L, Yasuda HK: Evaluation of plasma polymer-coated contact
lenses by electrochemical impedance spectroscopy. J Biomed Mater
Res 2001; 54: 597-607.
22. Pritchard N,
Fonn D, Weed K: Ocular and subjective responses to frequent replacement
of daily wear soft contact lenses. CLAO J 1996; 22: 53-59.
23. Gellatly K, Brennan N, Efron N: Visual decrement
with deposit accumulation on HEMA contact lenses. Am J Optom Physiol
Opt 1988; 65: 937-941.
24. Mondino B,
Salamon S, Zaidman G: Allergic and toxic reactions in soft contact
lens wearers. Surv Ophthalmol 1982; 26: 337-344.
25.
Baines M, Cai F, Backman H: Adsorption and removal of protein bound
to hydrogel contact lenses. Optom Vis Sci 1990; 67: 807-810.
26. Bohnert JL, Horbett TA, Ratner BD, Royce
FH: Adsorption of proteins from artificial tear solutions to contact
lens materials. Invest Ophthalmol Vis Sci 1988; 29: 362-73.
27. Bontempo AR, Rapp J: Protein-lipid interaction
on the surface of a hydrophilic contact lens in vitro. Curr Eye
Res 1997; 16: 776-81.
28. Castillo EJ,
Koenig JL, Anderson JM, Jentoft N: Protein adsorption on soft contact
lenses. III. Mucin. Biomaterials 1986; 7: 9-16.
29.
Minarik L, Rapp J: Protein deposits on individual hydrophilic contact
lenses: Effects of water and ionicity. CLAO J 1989; 15: 185-188.
30. Fowler S, Korb D, Allansmith M: Deposits
on soft contact lenses of various water contents. CLAO J 1985; 11:
124-127.
31. Minno G, Eckel L, Groemminger
S, Minno B, Wrzosek T: Quantitative analysis of protein deposits
on hydrophilic soft contact lenses: I. Comparison to visual methods
of analysis. II. Deposit variation among FDA lens material groups.
Optom Vis Sci 1991; 68: 865-872.
32. Sack
R, Jones B, Antignani A, Libow R, Harvey H: Specificity and biological
activity of the protein deposited on the hydrogel surface. Invest
Ophthalmol Vis Sci 1987; 28: 842-849.
33.
Jones L, Mann A, Evans K, Franklin V, Tighe B: An in vivo comparison
of the kinetics of protein and lipid deposition on group II and
group IV frequent-replacement contact lenses. Optom Vis Sci 2000;
77: 503-10.
34. Jones L, Franklin V, Evans
K, Sariri R, Tighe B: Spoilation and clinical performance of monthly
vs three monthly disposable contact lenses. Optom Vis Sci 1996;
73: 16-21.
35. Garrett Q, Chatelier RC,
Griesser HJ, Milthorpe BK: Effect of charged groups on the adsorption
and penetration of proteins onto and into carboxymethylated poly(HEMA)
hydrogels. Biomaterials 1998; 19: 2175-86.
36.
Garrett Q, Garrett RW, Milthorpe BK: Lysozyme sorption in hydrogel
contact lenses. Invest Ophthalmol Vis Sci 1999; 40: 897-903.
37. Garrett Q, Laycock B, Garrett RW: Hydrogel
lens monomer constituents modulate protein sorption. Invest Ophthalmol
Vis Sci 2000; 41: 1687-95.
38. Leahy C,
Mandell R, Lin S: Initial in vivo tear protein deposition on individual
hydrogel contact lenses. Optom Vis Sci 1990; 67: 504-511.
39. Jones L, Evans K, Sariri R, Franklin V, Tighe
B: Lipid and protein deposition of N-vinyl pyrrolidone containing
group II and group IV frequent replacement contact lenses. CLAO
J 1997; 23: 122-126.
40. Maissa C, Franklin
V, Guillon M, Tighe B: Influence of contact lens material surface
characteristics and replacement frequency on protein and lipid deposition.
Optom Vis Sci 1998; 75: 697-705.
41.
Jones L, Senchyna M, Louie D, Schickler J: A comparative evaluation
of lysozyme and lipid deposition on Etafilcon, Balafilcon and Lotrafilcon
contact lens materials. Invest Ophthalmol Vis Sci 2001; 42: s593
#3186.
42. Senchyna M, Jones L, Louie
D, Forbes I, May C: Optimization of methodologies to characterize
lysozyme deposition found on balafilcon and etafilcon contact lens
materials. Invest Ophthalmol Vis Sci 2002; ARVO abstract # 3082.
43. McNally J, McKenney CD: A clinical look
at a silicone hydrogel extended wear lens. Contact Lens Spectrum
2002; 17: 38 - 41.
44. Court JL, Redman
RP, Wang JH, Leppard SW, Obyrne VJ, Small SA, Lewis AL, Jones SA,
Stratford PW: A novel phosphorylcholine-coated contact lens for
extended wear use. Biomaterials 2001; 22: 3261-72.
45. McKenney C, Becker N, Thomas S, Castillo-Krevolin C, Grant T:
Lens deposits with a high Dk hydrophilic soft lens. Optom Vis Sci
1998; 75: 276.
46. Bruinsma GM, van der
Mei HC, Busscher HJ: Bacterial adhesion to surface hydrophilic and
hydrophobic contact lenses. Biomaterials 2001; 22: 3217-24.
47. Jones L, Long J, Chen P: The impact of
contact lens care regimens on the in vitro wettability of conventional
and silicone-hydrogel contact lens materials. Invest Ophthalmol
Vis Sci 2002; ARVO abstract # 3097.
48.
Hart D, Tidsale R, Sack R: Origin and composition of lipid deposits
on soft contact lenses. Ophthalmol 1986; 93: 495-503.
49. Franklin V, Bright A, Tighe B: Hydrogel polymers and ocular
spoilation processes. TRIP 1993; 1: 9-16.
50. Tighe BJ, Jones L, Evans K, Franklin V: Patient-dependent and
material-dependent factors in contact lens deposition processes.
Adv Exp Med Biol 1998; 438: 745-51.
 |
|